Electrolyzer technology is mature and is currently the most reliable method to extract hydrogen from water. However, high capital cost and its reliance on expensive electricity and clean water are the main reasons why electrolyzers haven’t revolutionized the green hydrogen economy as everyone hoped. We are working with UCLA to develop breakthrough technologies to dramatically reduce the capital cost of electrolyzers.
Until there is a new technology that does not rely on electricity as the input energy to make hydrogen, electrolyzers will continues to serve as an important bridge to the green hydrogen economy.


For more than 200 years, scientists have known how to split water into hydrogen (H2) and oxygen (O2). By placing two metal electrodes into a jar of salted water, an electrolytic solution, H2 and O2 will bubble up when an electrical voltage is applied. This process is called electrolysis and the device called an electrolyzer. However, to make this science work at commercial scale, expensive chemical catalysts are deposited on the electrode surface to lower the energy required to split water molecules.
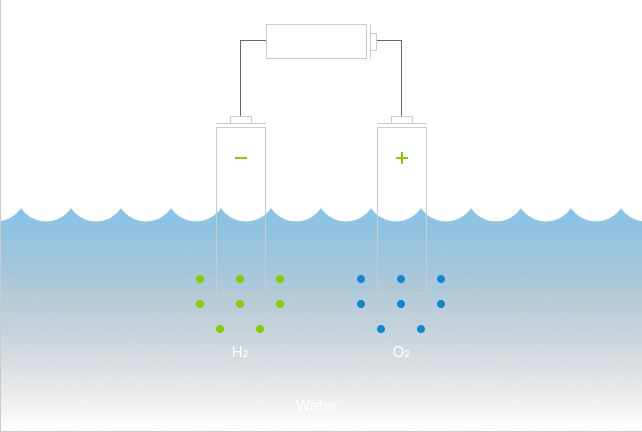
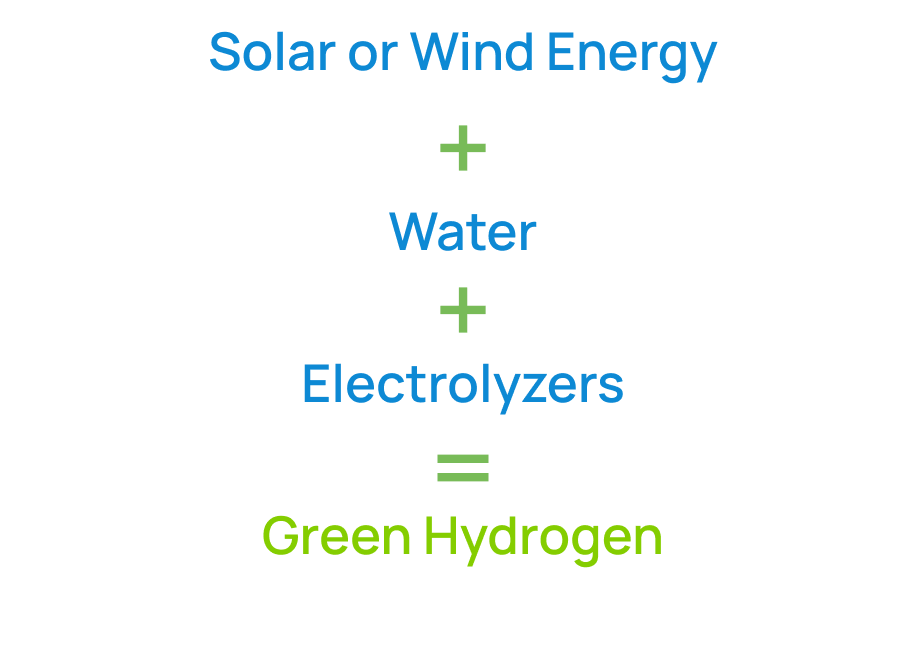
If the source of electricity is renewable such as solar or wind, then the resulting hydrogen is a zero-emission resource.
The most common types of electrolysis today are alkaline and proton exchange membrane (PEM). Alkaline electrolysis is the oldest and most mature electrolyzer technology and benefits from a low manufacturing cost and large-scale applications. However, this technology has the disadvantages of slower start-up, corrosion, complicated maintenance, large size, liquid electrolyte and many components in the device.
PEM electrolysis is a newer technology and has the advantage of quickly reacting to fluctuating input energy, such as from solar and wind. Its smaller footprint also makes it ideal for distributed production of hydrogen.
Electrolyzers are expensive because many rely on rare earth materials such as platinum and iridium as chemical catalysts for the water splitting reactions. Iridium is literally stardust found only in asteroids. Platinum is so rare that only 200 tons are mined every year and its demand is ever increasing in applications such as batteries, fuel cells, fiber optics, LCD displays, cancer treatment and many others. According to the National Renewable Energy Laboratory (NREL), these materials account for nearly 50% of the capital cost of PEM electrolyzers. Additionally, the cost of electricity contributes to over 50% of hydrogen production costs.
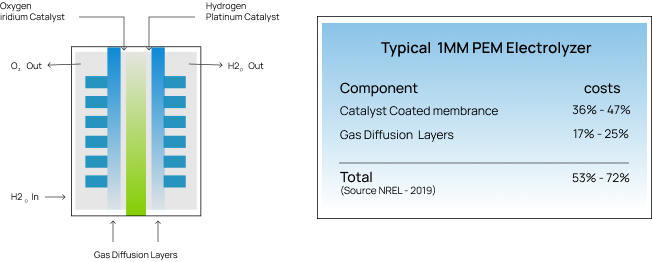
NewHydrogen is currently developing two distinct catalyst technologies to improve Alkaline and Proton Exchange Membrane (PEM) electrolyzers through materials cost reductions (CAPEX) and electrical efficiency improvements (OPEX).
In alkaline electrolysis, oxygen generation is fast and easy, but hydrogen generation is slow and limits the efficiency of the entire water splitting reaction. Using platinum as the hydrogen catalyst can overcome this problem, but at a very high cost. We are developing breakthrough hydrogen catalysts that can reduce the use of platinum by more than 10X, as well as a novel single-atom non-precious metal catalyst to replace platinum all together.
In PEM electrolysis, hydrogen generation is fast and easy, but oxygen generation is slow, which limits the efficiency of the entire water splitting reaction. Iridium is the material of choice for PEM oxygen generation, but it is even more rare than platinum. We are developing a breakthrough oxygen catalyst based on non-precious metals to replace iridium all together. Additionally, the low-platinum or no-platinum hydrogen catalysts we are developing for alkaline electrolysis can also be used in PEM electrolysis to incrementally lower the device cost as well.
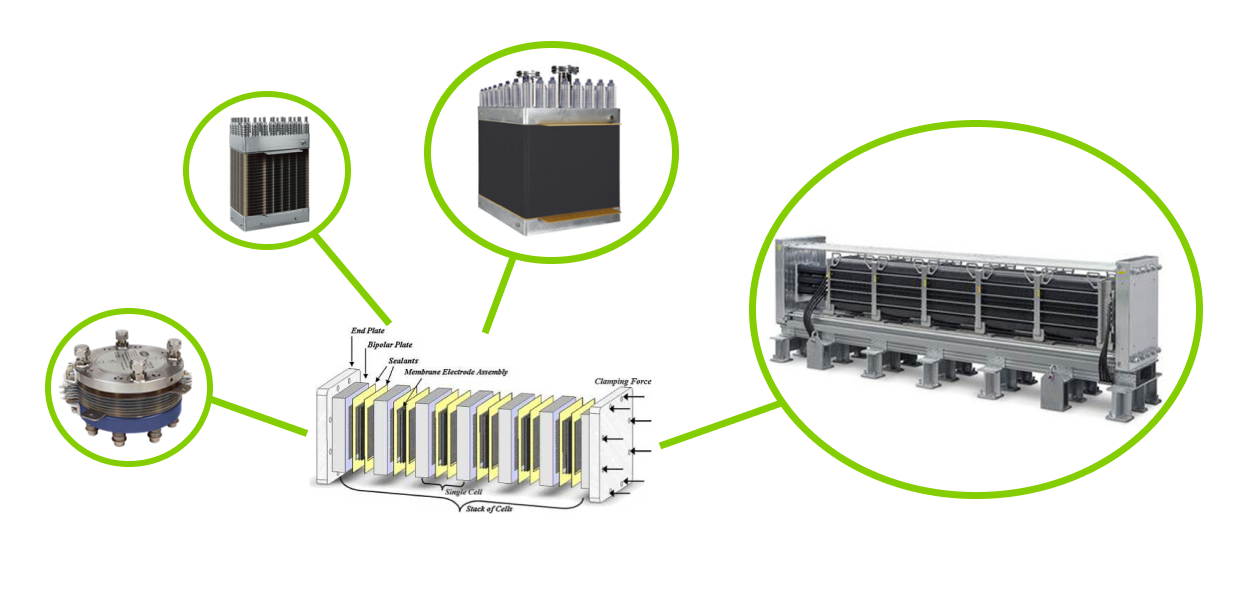
Due to the inherent architecture of electrolyzers, they are highly scalable to meet the demands of many applications. Once a breakthrough single electrolyzer cell is developed, any size system can be built by simply stacking up cells and stringing up systems to meet any demand.
The evolutionary cost reduction of electrolyzers will require breakthroughs in multiple areas.
The International Renewable Energy Agency has identified these cost reduction
opportunities that we are also studying and exploring.
| CHALLENGE | BENEFIT | |
|---|---|---|
| High catalyst surface area > 50m/g | EASY | MEDIUM |
| High catalyst utilization > 80% | MODERATE | MEDIUM |
| Improved kinetics for both hydrogen and oxygen evolution with novel nickel-based alloy | MODERATE | HIGH |
| Mitigate catalyst poisoning/deactivation by foreign elements from electrolyte, and components present in the system | MODERATE | LOW |
| Design, create and integration form of recombination catalysts for gas permeation (crossover) | MODERATE | MEDIUM |
| Mitigate critical deration of catalysts on the anode side to avoid loss of surface area | DIFFICULT | HIGH |
| Mitigate nickelhydorgen (NiH) formation on the cathode side | DIFFICULT | LOW |
| Eliminate mechanical degradation of catalyst layers (delamination, dissolution) | DIFFICULT | HIGH |
| identify stable polymer chemistry that can be used as ionomer (OH - transport) to be used to fabricate electrodes for alkaline electroliers | DIFFICULT | HIGH |
| Identify and reduce interface resistance from catalyst layer to PTLs | DIFFICULT | HIGH |

| CHALLENGE | BENEFIT | |
|---|---|---|
| Mitigate membrane poisoning/deactivation by foreign elements from components and system | EASY | MEDIUM |
| Design, create and integration form of recombination catalysts for gas permeation (crossover) | EASY | MEDIUM |
| Increase catalyst utilization of anode and cathode catalysts | MODERATE | HIGH |
| Identify and reduce interface resistances from catalyst layer to PTLs | MODERATE | MEDIUM |
| Reduce the ohmic losses and gas permeation of PFSA membrances | DIFFICULT | HIGH |
| Improve kinetic for oxygen evolution using iridium-free catalysts and maintain stability comparable to iridium SoA | DIFFICULT | HIGH |
| Eliminate mechanical degradation of catalyst layers (delamination, dissolution) | DIFFICULT | MEDIUM |
| Create noble metal free protective layers for PTLs | DIFFICULT | HIGH |
| Create titanium free PTLs | DIFFICULT | HIGH |

Since 2020 we have been working with University of California at Los Angeles under aa sponsored research agreement to develop these electrolyzer catalyst technologies. During the term of the agreements, we have first rights to negotiate an option or license to all resulting intellectual property and related technology.
Our catalyst research and development program is led by Dr. Yu Huang, under a sponsored research program at the Department of Materials Science and Engineering at the University of California at Los Angeles (UCLA). From 2016-2020, UCLA was recently ranked the #1 public university in the nation by U.S. News & World report. The Materials Science and Engineering (MSE) department’s most active research areas include energy harvesting and storage, solar cells, materials for clean energy, micro/nano electronic devices, optoelectronics, graphene, nanowires, bio-inspired nanostructured materials, electroactive materials, nanocomposites, multifunctional materials, novel materials for infrastructure repair and retrofit, and archaeological materials. In support of these activities, MSE has established state-of-the-art resources in materials processing and characterization to facilitate research.

