
Since the invention of the internal combustion
engine in the 1870s, a new world order of
geopolitics and economic dependence was
uncomfortably forged between energy
producing countries and energy consuming
countries. This new world order propelled
humanity into an era of prosperity and
ambition, as well as an era of climate change
and instability where a select few nations
control natural resources such as oil, gas and
coal.
What if countries of the world can
independently produce their own fossil fuel
replacement, and not rely on uncertain or
unpredictable partnerships?
What if this new form of energy is as cheap as
oil and natural gas, but without greenhouse
gas emissions?
We believe cheap green hydrogen is that new form energy and it has the potential to shift global energy
supply from the hands of the few, to the hands of the many, democratizing energy and ushering in another
New World Order worth $12 trillion, as estimated by Goldman Sachs.
We believe that cheap green hydrogen is the new hope for a new era of peace and prosperity for all of
humanity. The International Renewable Energy Agency (IRENA) seems to agree.
For more than 100 years, the gold standard for producing green hydrogen is through electrolysis, using electrolyzers with solar or wind energy to split water into hydrogen and oxygen. However, electrolyzers are very expensive and their efficiencies are fundamentally limited by the natural laws of thermodynamics. For example, the theoretical voltage required to split water is 1.23V, but in real life, the voltage required in an industrial electrolyzer is closer to 2V, sometimes more. This 60% or more of additional energy is wasted and not put into hydrogen molecules.
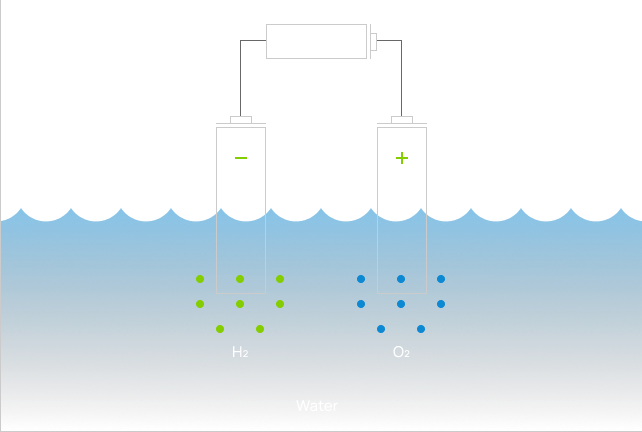
The electrolyzer was first invented in 1789 and its basic chemistry and architecture hasn’t changed much since then, despite many materials and manufacturing advancements. Nearly all electrolyzers suffer from the following disadvantages:
The need for much higher voltage, or input energy, to drive meaningful amounts of hydrogen production.
Catalysts used for water splitting are often precious metals such as platinum and iridium, a material so rare it can only be found in asteroids, and they all corrode over time.
Degradable membranes are needed to separate hydrogen (H2) and oxygen(O2) bubbles so they don’t re-combine to make water (H2O).
Precious metals and membranes are highly susceptible to fouling, therefore expensively distilled pure water is required.
Water splitting reactions can only happen on the surfaces of 2-dimentional electrode plates. Therefore, much of the water is literally waiting around to be zapped, resulting in low efficiency and low throughput.
The biggest problem with electrolyzers is the use of electricity, which accounts for nearly 73% of the cost of Hydrogen production.
Oxford Institute for Energy Studies (2022)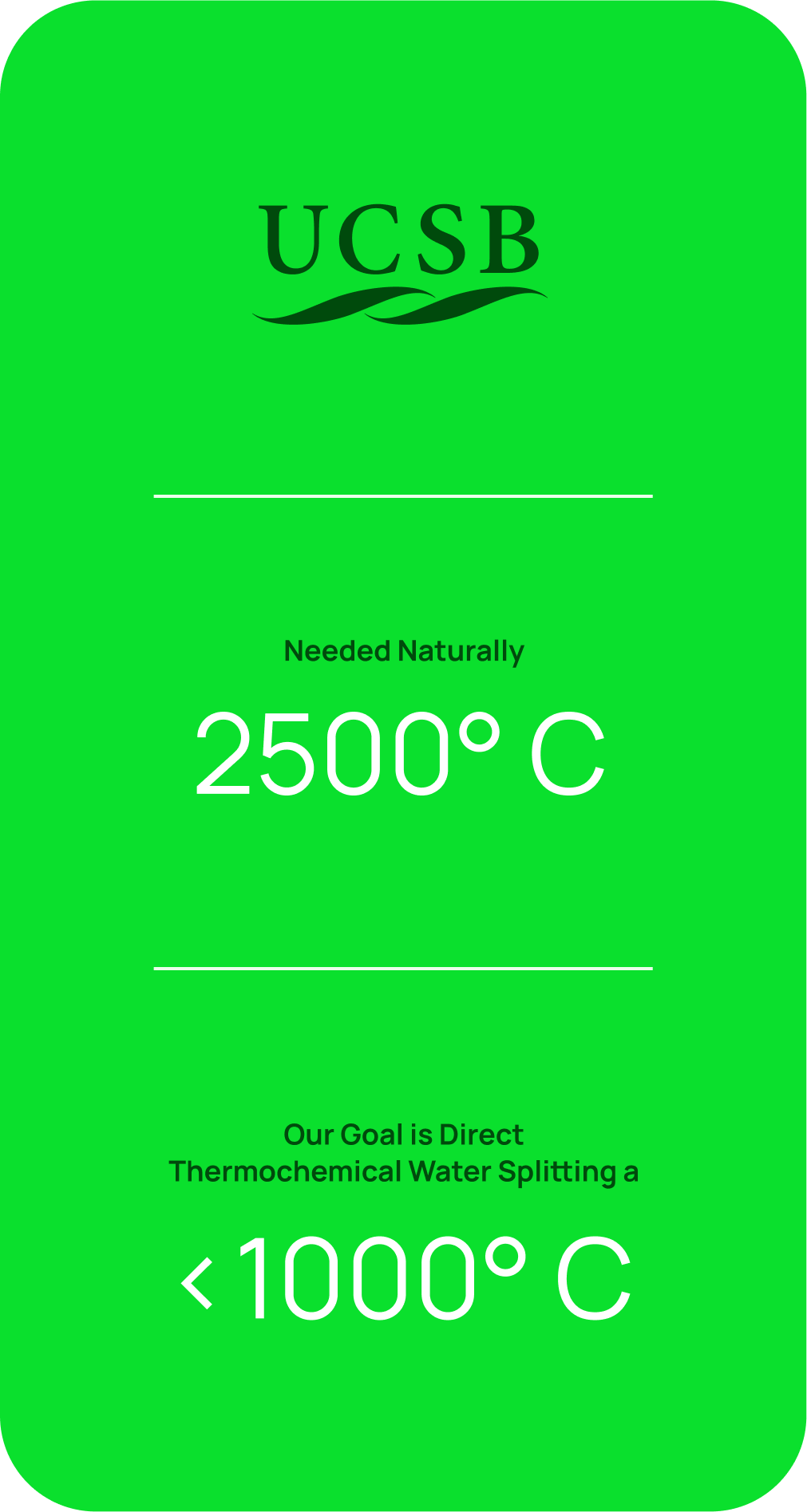

Working with a team of world-class chemical and materials engineers at UC Santa Barbara (UCSB), we are developing a better way to efficiently split water into cheap green hydrogen with a thermochemical approach, using heat instead of electricity. The UCSB team plans to exploit the oxidation reduction features of multi-component materials including high temperature liquids to directly split water continuously in a series of chemical looping reactions, producing hydrogen and oxygen in separate reaction chambers. The concept has been at work in other applications for some time at UCSB and we decided to sponsor research to apply the methodology to water splitting.

It is known that water spontaneously splits into hydrogen and oxygen at temperatures in excess of 2,500°C with near 100% efficiency. However, such temperatures are not easily achievable or manageable. Despite its alluring efficiency, there are currently no thermochemical water splitting hydrogen production systems working at large production scale, only small and lab scale.

The UCSB team is developing a breakthrough technology to exploit the features of a Molten Catalytic Liquid to directly split water continuously in a single redox chemical loop, to produce hydrogen and oxygen in separate chambers. Our novel Molten Catalytic Liquid is reduced in one chamber, oxidized in another chamber, and is continuously recycled and reused. The only inputs are heat and water. This will be a novel, first of its kind, high efficiency thermochemical water-splitter that uses low cost common materials and common industrial temperatures of less than 1,000oC, to potentially produce the world’s cheapest green hydrogen.

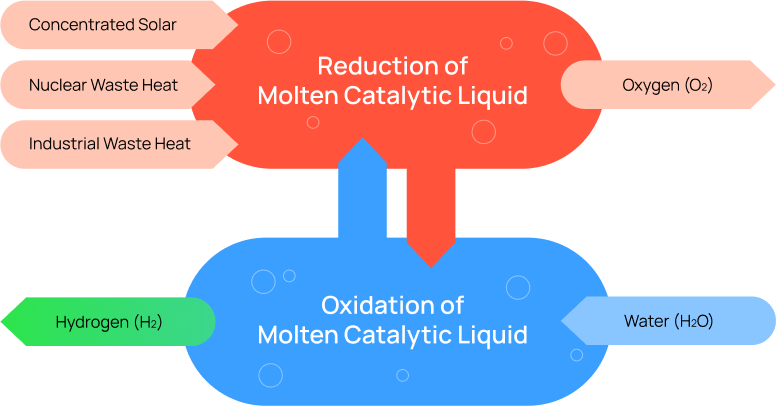
Our novel NewHydrogen 
Most electricity is made from the inefficient burning of fossil fuels to boil water, to make steam, to turn a turbine, which produces electricity. Or from solar panels that can only convert 20% of solar energy into electricity. We go straight to the source, heat, and bypass expensive and inefficiently generated electricity.
Unlike the 2D reaction surfaces of electrolyzers, the entire volume of the reactor chamber filled with our novel Molten Catalytic Liquid are reaction surfaces. Water/steam molecules everywhere inside the reactor are instantly split into hydrogen and oxygen. The result is a high speed and high throughput water-splitting process.
The novel Molten Catalytic Liquid used in this process are made from very low-cost materials and doubles as the intermediary redox compound, as well as the catalyst for water splitting.
Since hydrogen (H2) and oxygen(O2) gas are created in different reaction chambers, there is no need for membranes.
A thermal system is fundamentally different and more efficient than an electricity based one, with no minimal voltage requirement.
Any source of water can be evaporated into input steam for our process. Therefore, expensively distilled water is not required.
Electrolyzers are made with many parts stack together with many points of failure. The NewHydrogen ThermoLoop design is a conventional low cost, highly scalable, industrial process.
 A Better Form of Electrolyzer
A Better Form of ElectrolyzerElectrolyzers require electricity, a fundamentally less efficient form of energy and projected to
be 60% the cost of producing green hydrogen even by 2030. Most electricity is made from the
inefficient burning of fossil fuels to boil water, to make steam, to turn a turbine, which
produces electricity. Alternative sources of energy, such as sun and wind, are intermittent.
When the sun shines, solar panels only convert 20% of solar
energy into electricity. Wind turbines can produce electricity, but the wind is less predictable
than the sun. By using heat as the power source for the NewHydrogen ThermoLoop, we cut out the
middleman – electricity – and go straight to the heat
source. Concentrated solar heat, nuclear waste heat, or industrial waste heat are extremely low
cost compared to electricity and can be used to power our ThermoLoop process.
In areas where electricity is more readily available than high temperature heat, we envision the
use of a readily available high efficiency electric heater to supply heat to the NewHydrogen
ThermoLoop process. While our ThermoLoop may look like a standard electrolyzer, the similarities
stop there: it will be faster, cheaper, simpler, and more efficient.
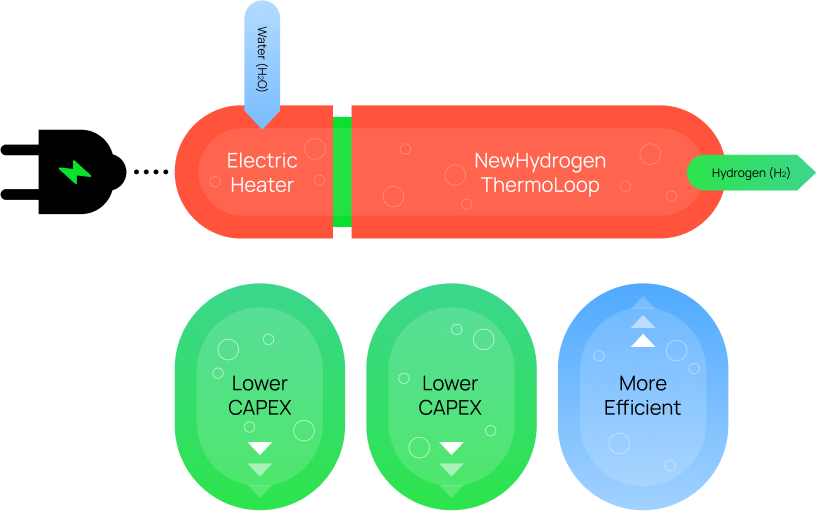
The UCSB team plans to exploit the oxidation reduction features of multi-component materials including high temperature liquids to directly split water continuously in a series of chemical looping reactions, producing hydrogen and oxygen in separate reaction chambers. The concpet has been at work in other applications for some time ay UCSB and the Company decided to sponsor research to apply the methodology to water splitting.
The research team at UCSB is led by Dr. Philip Christopher, an expert in reaction engineering and
catalysis. The UCSB team has extensive of experience working with molten metals and molten liquids
chemistry, as well as extracting “blue” hydrogen from natural gas with carbon capture using molten
metals. UCSB is ranked one of the top research universities in the world and a global leader in
bioengineering, chemical and computational engineering, materials science, nanotechnology and
physics. UCSB boasts 6 Nobel Laureates (five in sciences and engineering) and one winner of the
prestigious Millennium Technology Prize. According to Times Higher
Education's 2021 World University Rankings, UCSB is ranked top 2 percent of the engineering programs
in the world in overall excellence, research influence, and innovation.
The ThermoLoop technology is currently in its early development stage. In June 2023, we entereded into a sponsored research agreement with the University of California Santa Barbara (UCSB) to perform this research and development work. During the term of the agreement, we have rights to negotiate an option or license to all resulting intellectual property and related technology.




Electrolyzers are the current gold standard for producing green
hydrogen. However, despite all the incremental improvements
since its invention in 1789, electrolyzers have not changed
the input cost of electricity and clean water.
Rethink Energy estimates that the cost of electricity and clean water (fuel cost) as a
percentage of the total cost of electrolyzer hydrogen production will grow from
50%
today to 75% by 2032.

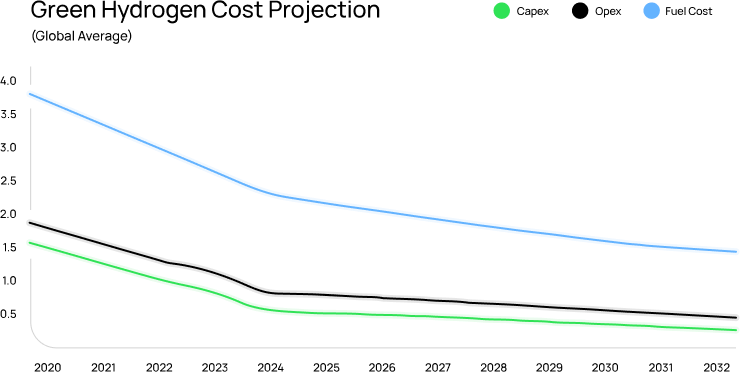
NewHydrogen’s ThermoLoop™ would eliminate
the need for electricity and clean water